Problems With Irb System
Problems with irb system. An institutional review board IRB also known as an independent ethics committee IEC ethical review board ERB or research ethics board REB is a type of committee that applies research ethics by reviewing the methods proposed for research to ensure that they are ethicalSuch boards are formally designated to approve or reject monitor and review biomedical and behavioral research. The IRB records must include documentation of the date when the IRB chairperson or other individuals designated by the IRB determined that all conditions of IRB approval have been satisfied and the approval becomes effective and the expiration date of the initial IRB approval ie the date by which the first continuing review must occur 45 CFR 46102h and 46115a. The BSDUCMC IRBs are charged with the responsibility for review approval and surveillance of research involving human subjects carried out in the BSD and the University of Chicago Medical Center.
The following problems can be solved by following the 3 steps above. IRBCSS also answers questions relating to the electronic filing of Forms 8955-SSA. Use of this system is subject to Stanford Universitys rules and regulations.
Proceed to complete the application Please make certain to complete all entries. The consent form must include the same required elements as a consent form where another IRB serves as the reviewing IRB CHOP ICF Requirements. If you have general questions regarding Buck-IRB please contact Michael Donovan Buck-IRB Liaison at 614-292-6950 or donovan6osuedu.
Please contact the IRB office with questions at 517-355-2180 or irbmsuedu. A researcher sets up a meeting with the superintendent of a large and diverse public school system to get data about the ethnic composition of the school system and the number of students. The Office for the Protection of Research Subjects OPRS provides administrative support for the review and approval of research protocols experiments involving humans and human embryonic stem cells hESWe help ensure that regulations are adhered to for the protection and welfare of subjects investigators and the University.
Submission of new Expedited and Full Review applications in E-IRB became mandatory 1222018. The IRB is a committee appointed to ensure rights safety and welfare of human research subjects. See the Stanford Administrative Guide for more information.
SystemBrowser Requirements. Start studying CITI IRB Populations and Unanticipated Problems. If you experience a technical issue like problems with logging into Click please contact the Click Help Desk at 517-355-2000 or clickhelpdeskmsuedu.
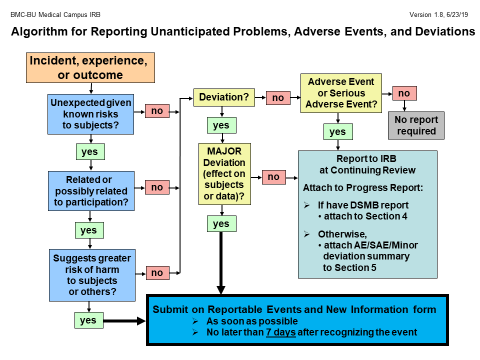
The University of Massachusetts-Amherst UMass-Amherst IRB requires faculty staff and students graduates and undergraduates who are conducting or part of a research group to take the research-appropriate course on the CITI Program Webpage and complete certification before submitting the Kuali protocol for initial approval amendment renewal to be approved by the IRB. Determining which adverse events should be considered unanticipated problems warranting submission to the IRB.
See the Stanford Administrative Guide for more information.
This guide contains screen shots of myIRB and step-by-step directions for using the system. Use of this system is subject to Stanford Universitys rules and regulations. Proceed to complete the application Please make certain to complete all entries. Technical problems with the Buck-IRB system can be directed to ORhelpdeskosuedu. If you experience a technical issue like problems with logging into Click please contact the Click Help Desk at 517-355-2000 or clickhelpdeskmsuedu. Name title and the site or institutional affiliation of the person authoring the CAPA Issue. E-IRB is the University of Kentuckys Link Blue-secure web-based system used to submit human research applications and Other Reviews to the Institutional Review Board IRB for review and approval. Determining which adverse events should be considered unanticipated problems warranting submission to the IRB. REQUIRED TRAINING for myIRB.
If you experience a technical issue like problems with logging into Click please contact the Click Help Desk at 517-355-2000 or clickhelpdeskmsuedu. 19-01-1939 select desired action continuing review amendment final report adverse event etc. If you experience a technical issue like problems with logging into Click please contact the Click Help Desk at 517-355-2000 or clickhelpdeskmsuedu. Do not use myIRB if you are submitting to IRB-04 aka WIRB. The Declaration of Helsinki introduced the concept of an independent committee which evolved into the institutional review board IRB system used in the US. If you wish to practice using the system prior to creating a research protocol please visit the myIRB Sandbox If you have general research questions please contact the. SystemBrowser Requirements.
























Posting Komentar untuk "Problems With Irb System"